An Israeli company’s groundbreaking heart pump technology has given its first recipient a new lease on life.
By: CTech
In December, doctors at the National Research Center for Cardiac Surgery in Astana, Kazakhstan, performed a first of its kind surgery on 24-year-old Ismail Tursunov. They implanted him with a left ventricular assist device (VAT), essentially a mechanical pump intended to help the heart pump oxygenated blood from the lower chambers of the heart to the body. This type of procedure is performed in thousands upon thousands of patients every year, but this time something was different. Tursunov was the first human implanted with a wirelessly charged pump.
The pump was manufactured by Jarvik Heart Inc., a market veteran that developed the first artificial heart successfully implanted in a human. The technology for charging the battery that powers it, however, was developed by a relative newcomer: an Israel-based company called Leviticus Cardio Ltd., founded in 2008.
VATs are a last resort for people that are either awaiting a heart transplant, or are not eligible or good candidates for one. More rarely, they are used to buy people time until their hearts heal enough to beat on their own. VATs rely on an external power source, which currently means a permanent exit wound is left in the skin for a wire to go through, running an increased risk of infection and hindering activities such as swimming or even showering.
Wireless powering has been tried before by commercial companies and researchers, Leviticus Cardio director Steve Rhodes told Calcalist in a Thursday interview. The problem was, he explained, all prior attempts made use of the same technology now being used to wirelessly charge iPhones, and the amount of energy transferred basically “cooked” the tissue from within. Leviticus Cardio’s technology, he said, uses a very small amount of energy, and so far tests have not shown any resulting damage.
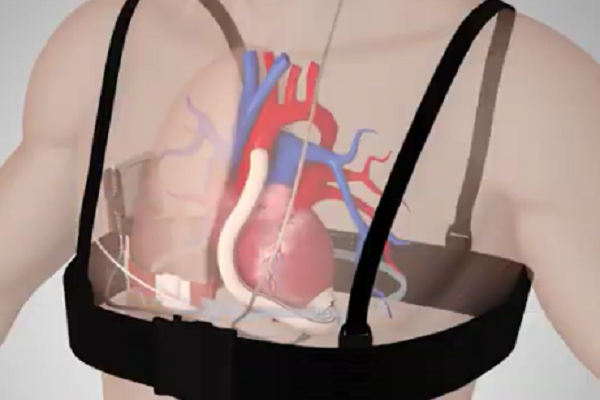
Helmed by CEO Michael Zilbershlag, Leviticus Cardio’s technology uses a receiver inductive coil implanted in the body alongside the VAT, coupled with a battery and an internal controller. An external coil, placed within a vest for easy carrying, charges the inner coil electromagnetically. The battery lasts for around eight hours, after which the user needs to wear the vest for charging. A wrist monitor is used to keep track of the VAT, coupled with an internal vibration alarm that is triggered by a major hazard such as low battery power.
In case of malfunctions, Tursunov has also been implanted with a wire-based backup charging system that uses an internal power cable connected to the VAT. The cable passes under the neck skin and provides a skull-mounted “socket” behind the ear, in the same area cochlear implant electrode wires are passed into the body. The technology, also developed by Jarvik, is already approved and in use in Europe, but Leviticus Cardio states it has not been needed once since the surgery.
Currently, the technology is intended as a temporary solution before a heart transplant, but Rhodes said the company is looking at the technology as a permanent solution in the future.
There are always going to be more patients in need of a transplant than available hearts, Rhodes said. “I expect that at least initially, the patients we implant will be a ‘bridge to transplant,’” he said. Once the benefits and safety of the technology are made apparent, he expects it will become a long-term treatment.
The main problem with permanent VATs today, Rhodes explained, is the continuous risk of infection. “Wireless technology could be the solution.”
In January, Leviticus Cardio and Jarvik received a $950,000 grant from the Israel-US Binational Industrial Research and Development (BIRD) Foundation. Tursunov was the “first in human” test, Rhodes said, and now the company plans to use the funding to update the product, start clinical trials, and get the product to market in both the US and Europe within two to three years.
Watch a video on the new technology below:
Do You Love Israel? Make a Donation - Show Your Support!
Donate to vital charities that help protect Israeli citizens and inspire millions around the world to support Israel too!
Now more than ever, Israel needs your help to fight and win the war -- including on the battlefield of public opinion.
Antisemitism, anti-Israel bias and boycotts are out of control. Israel's enemies are inciting terror and violence against innocent Israelis and Jews around the world. Help us fight back!